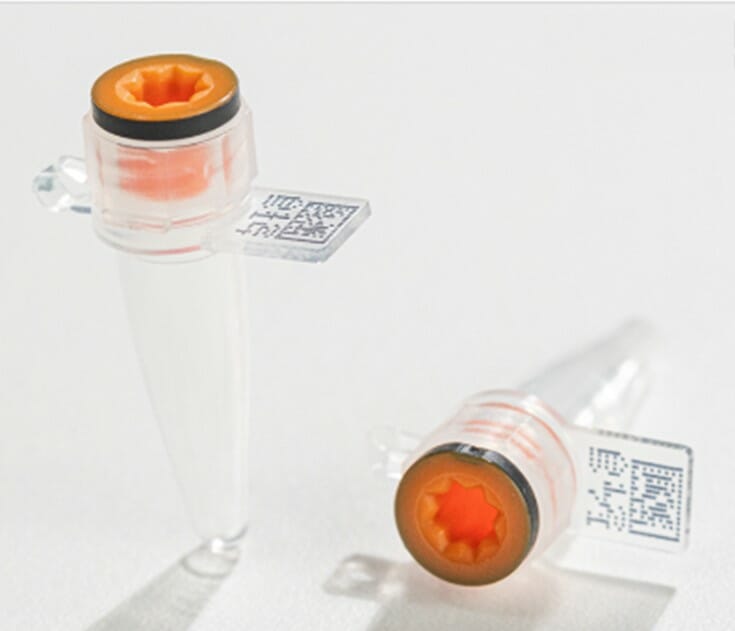
Azenta Life Sciences has launched Cap2TM – a new 0.2ml PCR microcentrifuge tube featuring a novel dual-cap design with both hinged lid and screw cap to preserve the integrity of samples used in genetic testing workflows. Acting as both a collection and processing tube, sample transfer can be minimized making for greater efficiency and less wastage.
Designed to ensure sample traceability in wide ranging genetic testing workflows, including pre-implantation genetic testing (PGT) – each Cap2 tube has a unique 2D datamatrix code as well as a numerical human readable identifier to prevent sample tracking errors.
All Azenta tubes, including the new Cap2, are developed with broad compatibility in mind, performing without compromise in conjunction with automated code reading, capping and sample management systems from Azenta and all other industry-recognized manufacturers.
Manufactured from high quality virgin polypropylene, in a class 8 clean room environment, each batch of tubes is tested to certify them endotoxin and DNase/RNase free as well as containing no detectable leachables or extractables. Vital considerations for sample collection tubes used in genetic testing workflows.
To learn more about how to improve sample tracking, ensure sample integrity and drive process efficiency with the Cap2 PCR tube please visit https://www.azenta.com/products/0.2ml-dual-cap-sample-collection-pcr-tube
Azenta is a market leader in the design and manufacture of consumables and bench top instrumentation for a wide range of life science and medical applications, including research into combatting cancer and infectious diseases, drug development, molecular diagnostics, and forensics. Our leading capabilities across genomics, cryogenic storage, automation, and informatics is dedicated to sample exploration and management. We help our customers bring impactful breakthroughs and therapies to market with greater speed and precision.







